
And we suddenly introduce a stress such as we increase theĬoncentration of reactant A. The hypothetical reaction where gas A turns into gas B. Of a reactant or product is one way to place a stress
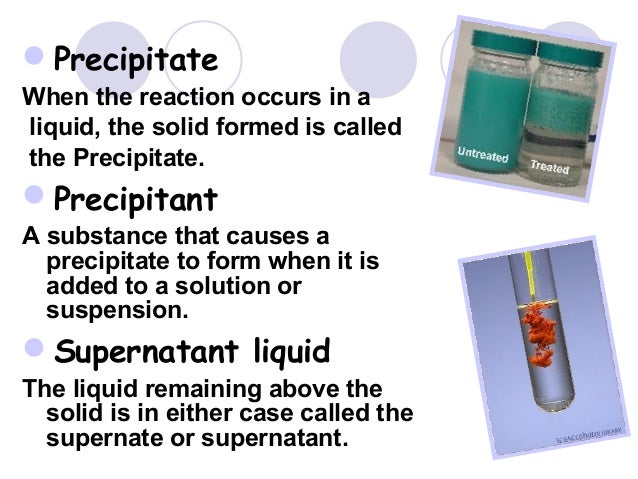
Reaction mixture at equilibrium, the net reaction goes in the direction that relieves the stress. Note, the equilibrium does not necessarily imply that the concentrations are the same, that may or may NOT be the same.Ĭhatelier's principle says, if a stress is applied to a For example, if you put a catalyst into the system, the equilibrium is not effected, but the reaction takes less time to happen because the Ea (the energy that is needed to be enough for the rxn) has been decreased, so you have increased the forward and reverse equally (both in magnitude and direction). Equilibrium also depends on things like temperature, pressure (which, according to Boyle's Law and KMT, is inversely proportional to volume), total pressure (even of non-reacting gas) and catalysts. If I increase the concentration of diatomic nitrogen gas, the system will most likely shift right because the best way to correct that stress you caused and more or less restore a sort of balance is to make more ammonia. Take the Haber-Botsch Process to manufacture ammonia. Le Châtlier's Principle pretty much just says that if I disturb a system that has this equilibrium, the system will try to restore a 'balance' by shifting the reaction in the direction to minimize the stress you caused on the system. In essence, it is pretty much just balance of chemical reactions in the system. In other words, reactants and products coexist in the chemical system. There is no net change in product nor reactant. Let me see if my understanding is correct: Equilibrium is pretty much when the rate of forward reaction is equal and opposite to rate of the reverse reaction.


 0 kommentar(er)
0 kommentar(er)
